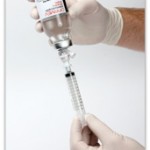
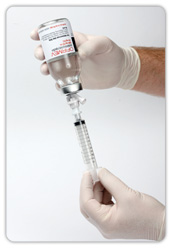
 First and only IV acetaminophen in the US
First and only IV acetaminophen in the US
- Indicated for the management of mild to moderate pain; management of moderate to severe pain with adjunctive opioid analgesics; and reduction of fever
- For adults and children ≥2 years old2
Comprehensive clinical dataset
- The approval of OFIRMEV is supported by the results of 20 clinical trials involving 1375 patients1,2
- IV acetaminophen has been studied in randomized clinical trials across a variety of surgical contexts and patient types1,3-5
Improved pain relief, reduced opioid consumption3
- In total hip/knee replacement surgery, OFIRMEV 1 g + PCA* morphine demonstrated significant pain relief and reduced opioid consumption compared to placebo + PCA morphine3
- The clinical benefit of reduced opioid consumption was not demonstrated
Patient satisfaction3
- The hip/knee replacement study also showed that OFIRMEV 1 g + PCA morphine significantly improved patient satisfaction compared to placebo + PCA morphine†3
Significant fever reduction1
- In adults with fever, antipyretic onset of action was significantly faster and duration of effect significantly longer with OFIRMEV than with placebo1
Established safety profile and well tolerated in clinical trials1-5
- OFIRMEV was shown to be well tolerated in clinical trials1-5
- The safety of IV acetaminophen has been monitored through a postmarketing surveillance program, with more than 400 million doses distributed since its European approval1
- IV acetaminophen has been used outside the US since 2002 and is approved in over 60 countries1
Dosing for adults and children ≥2 years old2
- OFIRMEV has a well-described pharmacokinetic profile2
- Dosing guidelines have been established for children ≥2 years old through adults2
- OFIRMEV is the first IV analgesic and antipyretic agent approved for children ≥2 years old2
Indication
OFIRMEV is indicated for the management of mild to moderate pain; the management of moderate to severe pain with adjunctive opioid analgesics; and the reduction of fever.
Important Safety Information
Do not exceed the maximum recommended daily dose of acetaminophen.
Administration of acetaminophen by any route in doses higher than recommended may result in hepatic injury, including the risk of severe hepatotoxicity and death.
OFIRMEV is contraindicated in patients with severe hepatic impairment, severe active liver disease or with known hypersensitivity to acetaminophen or to any of the excipients in the formulation.
Acetaminophen should be used with caution in patients with the following conditions: hepatic impairment or active hepatic disease, alcoholism, chronic malnutrition, severe hypovolemia, or severe renal impairment.
OFIRMEV should be administered only as a 15-minute infusion.
Discontinue OFIRMEV immediately if symptoms associated with allergy or hypersensitivity occur.
Do not use in patients with acetaminophen allergy.
The most common adverse reactions in patients treated with OFIRMEV were nausea, vomiting, headache, and insomnia in adult patients and nausea, vomiting, constipation, pruritus, agitation, and atelectasis in pediatric patients
The antipyretic effects of OFIRMEV may mask fever in patients treated for postsurgical pain.
To report SUSPECTED ADVERSE REACTIONS, contact Cadence Pharmaceuticals, Inc.
at 1-877-647-2239 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
References: 1. Data on file. Cadence Pharmaceuticals, Inc. 2. OFIRMEV™ (acetaminophen) injection prescribing information. 3. Sinatra RS, Jahr JS, Reynolds LW, Viscusi ER, Groudine SB, Payen-Champenois C. Efficacy and safety of single and repeated administration of 1 gram intravenous acetaminophen injection (paracetamol) for pain management after major orthopedic surgery. Anesthesiology. 2005;102:822-831. 4. Atef A, Fawaz AA. Intravenous paracetamol is highly effective in pain treatment after tonsillectomy in adults. Eur Arch Otorhinolaryngol. 2008;265:351-355. 5. Memis D, Inal MT, Kavalci G, Sezer A, Sut N. Intravenous paracetamol reduced the use of opioids, extubation time, and opioid-related adverse effects after major surgery in intensive care unit. J Crit Care. 2010;25:458-462.
*Patient-controlled analgesia.
†Subjects were asked to evaluate the study treatments, overall, using a 4-point categorical scale.
Adobe® Reader® is required to view PDFs. If your computer does not have it installed, download it free here.
This site is intended for US healthcare professionals only.
I have used this new drug several times and each time the patient was very comfortable in the recovery room. Obviously, I need more experience with the drug but so far it seems to work well.
Wow I must confses you make some very trenchant points.
I am glad to be a visitant of this great web site! , thanks for this rare information! .
And I tuhohgt I was the sensible one. Thanks for setting me straight.
I don’t know who you wrote this for but you helped a brtoher out.
Gee wilkliers, that’s such a great post!
Hello there! I could have sworn I’ve been to this website before but after reading through some of the post I realized it’s new to me. Nonetheless, I’m definitely glad I found it and I’ll be book-marking and checking back often!
I tried this drug and was surprised at how comfortable my patients were in the recovery room. It is a good option on a lot of patients.
A round of applause for your blog post.Really looking forward to read more. I have used Ofirmev and it works well. The only problem is that the bottle is too big to fit in our drug box so you have to get it separately. Also, I enjoy the fitness posts on this site.
Thanks for sharing, this is a fantastic article.Thanks Again.
I enjoy this piece of writing. I actually do Ofirmiv and find that my patients are more comfortable that when they receive Ketorolac. It is a good addition to our drug list.